Ask a GP or pharmacist for a low-egg or egg-free vaccine. Recombinant Influenza Vaccine RIV FluBlok RIV is a recombinant hemagglutinin influenza vaccine available as a trivalent formulation.

Influenza Vaccination Recommendations For 2017 2018 Updates From Acip Practice Guidelines American Family Physician
Compared with the Southern Hemisphere flu vaccine recommendation this recommendation represents one update and that is to the influenza AH3N2 component.

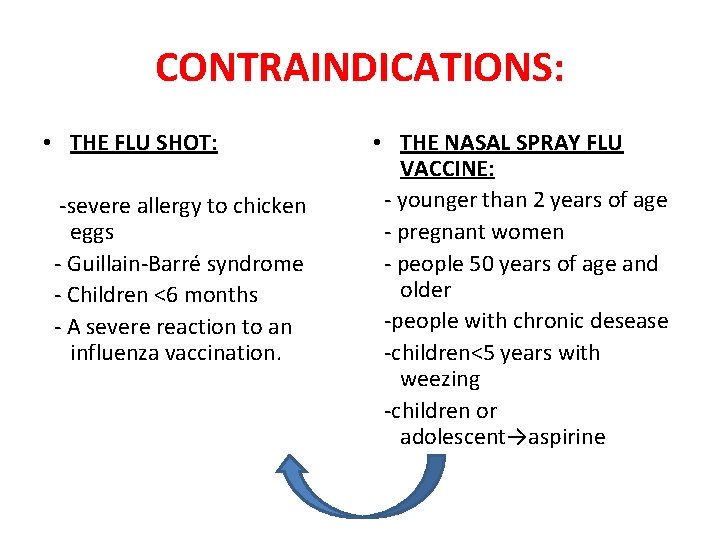
Influenza vaccine contraindications. Neurologic signs or symptoms following administration of an influenza vaccine is a contraindication to future use. Injectable influenza vaccine doses only contain small traces of formaldehyde. See Prevention and Control of Seasonal Influenza with Vaccines.
Wild-type influenza Children without contraindications or precautions to IIV or LAIV4 When choosing influenza vaccine for children 2 years of age without contraindications to IIV or LAIV4 we prioritize vaccine. Measles - in addition to the two absolute contraindications noted above measles vaccine is contraindicated in persons who are immunocompromised and in persons who are pregnant. The second columnlists individual vaccines when recommendations differ by vaccine.
Influenza - there are only the two absolute contraindications as noted above. Most adults can have the flu vaccine but you should avoid it if you have had a serious allergic reaction to a flu vaccine in the past. An egg-free recombinant hemagglutinin vaccine RIV may be used in people age 18 through 49 years with egg allergy of any severity who have no other contraindications.
You may be at risk of an allergic reaction to the flu vaccine injection if you have an egg allergy. This is because some flu vaccines are made using eggs. Tradename FluMist usually means no further doses.
Influenza Vaccine Contraindications Influenza Inactivated Injectable IIV Influenza Recombinant RIV Common Myth Severe allergic reaction eg anaphylaxis after a previous dose of RIV or to a vaccine component. Who Should and Who Should NOT get a Flu Vaccine. Contraindication to this seasons influenza vaccine.
RIV does not contain any egg protein. Allergic reactions to vaccinesis not a contraindication to receiving. Both the influenza AH1N1 and the influenza AH3N2 vaccine virus components were updated.
A formaldehyde allergy is usually not a contraindication to administering the influenza vaccine. RIV does not contain any egg protein CDC 2014. 12 Therefore it is important to reestablish the safety of.
Contraindications for use of CAIV-T influenza vaccine include anaphylactic reactions to eggs a history of Guillain-Barré syndrome patients aged. Adverse Effects and Contraindications for Flu Vaccines People who refrained from receiving the COVID-19 vaccine because of evolving safety data might express the same hesitancy toward the upcoming flu vaccines. For more information visit Influenza Vaccine for the 2021-2022 Season FDA external icon.
The Guide is arranged alphabetically according to symptoms and conditons that may correctly ornot be perceived as contraindications to vaccination. Contraindicated if ORS is present with lower respiratory involvement. Refer to Influenza Vaccine in Part 4.
33 filas Influenza vaccine. The first columnstates the symptom orcondition. Depending on the product at maximum 10 or 30 microgramsdose.
The third columnstates whether or not a person with that symptom or conditionshould be vaccinated. Vaccine Contraindications1 Precautions1 Hepatitis B HepB Severe allergic reaction eg. RIV is indicated for active immunization against disease caused by influenza virus subtypes A and type B and is approved for use in individuals 18 years of age and older.
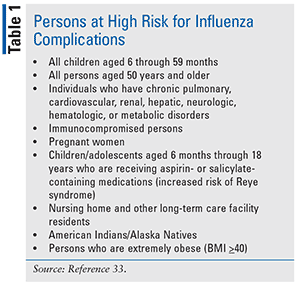
Everyone 6 months of age and older should get an influenza flu vaccine every season with rare exception. Annual influenza vaccination is recommended for all people aged 6 months and over unless medically contraindicated to reduce their chance of becoming ill with influenza. In addition to the contraindications based on history of severe allergic reaction to influenza vaccines noted in the Table each individual influenza vaccine is contraindicated for persons who have had a severe allergic reaction eg anaphylaxis to any component of that vaccine.
People with egg allergies who do not meet the age crite-. Anaphylaxis after a previous dose of any influenza vaccine anaphylaxis after any component of an influenza vaccine within 2 weeks of receiving a COVID-19 vaccination history of Guillain-Barré Syndrome whose first episode occurred within 6 weeks of receiving an influenza vaccine. Formaldehyde allergy is usually manifested as a delayed local irritation symptom on the skin.
For the 2019-20 flu season ACIP recommends annual influenza vaccination for everyone 6 months and older with any licensed influenza vaccine that is appropriate for the recipients age and. Mumps same contraindications as for measles vaccine. From 2020 all children aged 6 months to less than 5 years are newly eligible for free influenza.
Safe for most non-anaphylactic allergies with the following considerations. Recommendations of the Advisory Committee on Immunization PracticesUnited States 202021 Influenza Season for a list of contraindications and precautions for the nasal spray vaccine. Influenza vaccine should be withheld pending stabilization in patients with active neurologic disorders associated with changing neurological status.

Contraindications To Vaccines Download Table
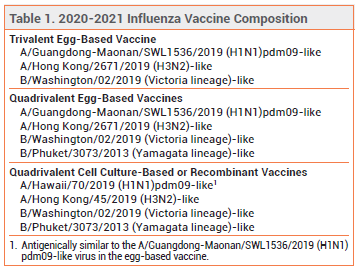
Influenza Vaccine For 2020 2021 The Medical Letter Inc
Http Dshs Texas Gov Idcu Disease Influenza Conference Docs 2017 Docs 2a Hoffman Influenza Vaccination 2017 Pdf
Flu Vaccination Medical Services Hse University

Influenza Vaccine For 2020 2021 The Medical Letter Inc
H 1 N 1 Influenza Vaccine Ana Mara

Naci Seasonal Influenza Vaccine Statement For 2019 2020 Ccdr 45 6 Canada Ca

Kuwait Flu Vaccine Booklet For Workshop
Tis The Season A Focus On Influenza

Pharmacovigilance Of A H1n1 Influenza Vaccines

Pdf Summary Of The Naci Seasonal Influenza Vaccine Statement For 2020 2021

Pharmacovigilance Of A H1n1 Influenza Vaccines

Influenza And Influenza Vaccine Ppt Video Online Download
Https Www Cambridgeshireandpeterboroughccg Nhs Uk Easysiteweb Getresource Axd Assetid 11059 Type 0 Servicetype 1

Pdf Vaccination In Multiple Myeloma Review Of Current Literature
Https Www Immunize Org Catg D P4067 Pdf


